We do not diagnose disease or recommend a dietary supplement for the treatment of disease. You should share this information with your physician who can determine what nutrition, disease and injury treatment regimen is best for you. You can search this site or the web for topics of interest that I may have written (use Dr Simone and topic).
“We provide truthful information without emotion or influence from the medical establishment, pharmaceutical industry, national organizations, special interest groups or government agencies.” Charles B Simone, M.MS., M.D.
40% HAVE A PFO (PATENT FORAMEN OVALE) – DO YOU?
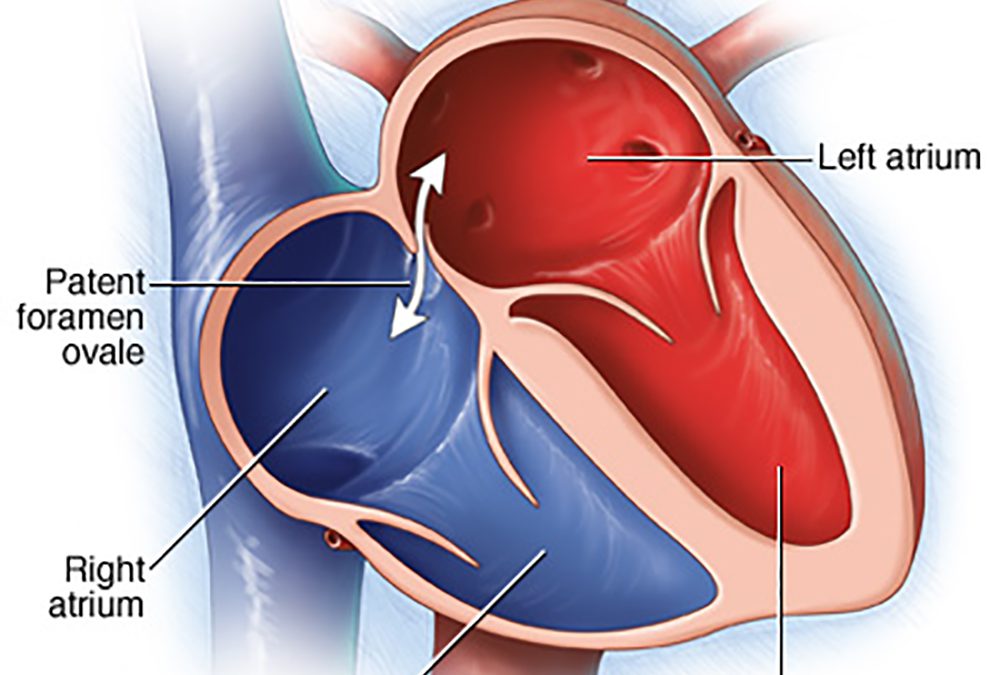
Lawrenceville, NJ (Dr Simone) – Do you get Jet Lag, fatigue, shortness of breath, chest pain, confusion, headaches, rapid heartbeat, or feel overheated, or have had an unexplained stroke, heart attack, or partial blindness? PFO (PATENT FORAMEN OVALE) affects up to 40% of all adults in the world and was first described by Claudius Galen in 200 A.D. It’s a hole in the upper chambers of the heart that usually closes during early infancy. Normally the blood travels from the right side of the heart through the lungs to the left side of the heart and then into the rest of the body before returning back to the right side of the heart. This allows the lungs to pick up oxygen and get rid of carbon dioxide (gas exchange), filter clots/particles out of the circulation, and cool the blood, and, therefore the body. A PFO has many other:
Important Actionable Clinical Physiological Consequences of a Patent Foramen Ovale (PFO) other than Stroke
Charles B. Simone, M.MS., M.D., Nicholas Simone Soule and Christina Simone Soule
Simone Protective Cancer Center
123 Franklin Corner Road, Lawrenceville, NJ 08648, USA, mail@drsimone.com


ABSTRACT
The work-up to detect a Patent Foramen Ovale (PFO) is usually done after a patient has a stroke. However, patients with PFOs may present with non-stroke signs and symptoms that have important actionable clinical physiological consequences not well known to be associated with a PFO or not considered, including hypoxia, carbon dioxide retention, higher core temperature that worsens hypoxia, high altitude effects with or without Altitude Decompression Sickness in a plane or mountain climbing, unfiltered fat emboli, unfiltered air bubbles, and SCUBA diving Decompression Sickness.
Some of these can negatively affect the quality of life, and cause profound medical changes, including death. This information, some of which is only recently available, presented succinctly and comprehensively for the first time, will provoke thoughtful discussion between physicians and patients with PFOs particularly for those whose PFOs get larger with aging. A suggestion can be made to close the foramen if there are no medical contraindications for closure.
(155 words) Key Words: PFO Patent Foramen Ovale; Stroke; Physiological consequences of PFO
INTRODUCTION
Patent Foramen Ovale (PFO) was first described by Claudius Galen in 200 A.D. In the 1984 classic study the prevalence of a PFO was reported to range from 25% to 35% identified using a probe during autopsy n=965 (1). Despite this range, 25% is often used and remembered to indicate the prevalence of PFO in adults. However, recent studies from several groups using saline contrast echocardiography report that conservatively 40% of adult humans have a PFO: 38% n=104 (2), 38% n=176 (3), 51.6% n=403 (4), and 53% n=45 (5). Shunting was present at rest and with provocative maneuvers (Valsalva, sniff, and/or cough) (4).
Every human fetus has a foramen ovale which is a hole in the wall between the upper right and left chambers of the heart (atria). This hole lets the blood bypass the fetal lungs which cannot function until they are exposed to air. At birth, the newborn breathes air and the foramen ovale closes. The hole seals completely within a few months for most people. Closure of the hole allows the blood to travel normally from the right side of the heart through the lungs to the left side of the heart and then into the rest of the body before returning to the right side of the heart. This allows the lungs to provide oxygen and get rid of carbon dioxide (gas exchange), filter clots/particles out of the circulation, and cool the blood, and therefore the body.
If the foramen ovale stays open it is called a patent foramen ovale and it can be small in some people and larger in others. No matter what the size of the foramen while resting, it becomes stretched and larger when the person sits or stands from a lying position, lifts something heavy, or bears down, as if having a bowel movement.
The incidence of PFO decreases with age, declining from 34% in people between the ages of 0 and 29 years to 25% in people between the ages of 30 and 79 years to 20% in people between the ages of 80 and 99 (1). However, the average size of the PFO increases with age from 3.4 mm during the first 10 years of life to 5.8 mm during the 10th decade of life (1).
Of the 795,000 strokes per year in America, the National Stroke Association indicates that approximately 100,000 are caused by PFO. Other studies indicate that PFO is detected in up to 40% of patients with embolic stroke of undetermined source [ESUS] (1,6,7,8).
However, no matter if it is 25% or 35% or 40% of all adults with PFOs on Earth, it is a massively large number of people who may present with non-stroke signs and symptoms that have important actionable clinical physiological consequences not well known to be associated with a PFO or not considered. The explanatory writing will enable laypersons to understand their many complex symptoms and signs. The information presented here will provoke thoughtful discussion between physicians and patients with PFOs to close the foramen if there are no medical contraindications for closure.
METHODS
To answer many complex questions, we investigated clinical medical and basic science journals, aerospace medicine publications, incorporated published Federal Aviation Administration (FAA) reports, interviewed thoughtful basic scientists, pituitary specialists, Aerospace Medicine physicians at Mayo Clinic and NASA, physician-scientists at the FAA, and physicians at the major hyperbaric centers at the University of Pennsylvania, Scripps, and Navy. This information is not readily available to many front-line clinicians that include cardiologists and neurologists, and specialists who focus on stroke/PFO.
IMPORTANT ACTIONABLE CLINICALPHYSIOLOGICAL CONSEQUENCES OF A PFO
At the end of a normal inspiration during diastole, the PFO allows a potentially significant amount of blood to bypass the lungs especially during exercise, at high altitude, and in patients with pulmonary hypertension. This does not occur with every heartbeat. That portion of the blood that does not go through the lungs will:
1) not pick up oxygen and get rid of carbon dioxide causing problems at rest and high altitude.
2) not be filtered of blood clots, air bubbles, or fat emboli. Consequently, unfiltered clots, air bubbles, and fat emboli can go directly to the brain (stroke or migraines with aura), heart arteries (heart attack), eye (blindness), kidney, any other organ.
3) not get cooled, resulting in a higher core temperature that causes an even lower oxygen level.
Patients with PFOs may present with the following non-stroke signs and symptoms.
REDUCED GAS EXCHANGE EFFICIENCY
A person with a PFO acquires less oxygen and retains more carbon dioxide for two reasons: 1) simply less blood flow to the lungs; and 2) gas exchange is less efficient (9). The size of the foramen matters. The larger the foramen, the less oxygen is picked up. Older patients (>65 years) with PFOs who also have heart failure with or without pulmonary hypertension have lower blood oxygen levels (SaO2) at rest (10). Surgical closure of the PFO improves arterial oxygenation at rest and during exercise, thus showing that blood flow through a PFO has a measurable impact on gas exchange (11).
Oxygen Saturation (SaO2)refers to the amount of oxygen picked up by red blood cells’ hemoglobin in the lungs. It is measured by a device called a pulse oximeter put on a finger. The normal range is 97% to 100%. Below 95% is considered low for people without diseases. Oxygen is usually administered if the level is about 92% or below. The normal range for a person with severe Chronic Obstructive Pulmonary Disease is 88%-92%.
Consequences of Hypoxia (Low Blood Oxygen)
1). Shortness of breath, fatigue, chest pain, changes in cerebral blood flow, confusion, headache, rapid heartbeat that results in decreased stroke volume. Oxygen blood levels below 80% can compromise the brain and heart. Continued low oxygen levels may lead to respiratory or cardiac arrest and loss of consciousness.
2). Constriction of small pulmonary arteries and dilatation of systemic arteries
3). Hypercoagulable state (increased by two-fold to eight-fold) results in clots that are caused by acute low-pressure hypoxia at high altitude (12).
4). Higher rate of death and morbidity for outpatients with pneumonia when their oxygen saturation is less than 90% (13).
5). Jet Lag is caused by this fall in oxygen saturation and altitude sickness.
BODY TEMPERATURE
When the core temperature rises in a person who does not have a PFO, the body responds by taking deeper breaths more rapidly to cool down the blood to maintain a constant brain temperature.
PFO patients have a higher core temperature at rest and during exercise (about 0.4 degrees C) because some of the blood does not go through the lungs to get cooled. This higher temperature results in an even lower oxygen level because of a right-shifted oxyhemoglobin dissociation curve. The SaO2 is reduced by 1.5% – a significant amount (9). Therefore, on a hot day in the sun, these patients can become fatigued and lethargic within minutes due to the higher core temperature and lower oxygen level without any significant blood pressure reduction. They have a higher risk for heat exhaustion and heat stroke. These patients feel better when they put at least their feet in a cooler body of water – pool, lake, ocean, even cool water in a bathtub. Air conditioning may help, but some have had difficulty cooling down when given cool air to breathe.
HUMIDITY
Adding humidity to the air decreases the oxygen molecules because the water molecules displace oxygen and the other gases. The total pressure of air at sea level is 760 mm Hg (mercury) with or without humidity. Nitrogen is the most abundant gas comprising 79% of air’s concentration, followed by oxygen at 20.93% and carbon dioxide at 0.03%.
The concentration of oxygen in air without humidity is calculated by multiplying 20.93% times 760 mm Hg = 159 mm Hg. This is the concentration of oxygen that enters the mouth and nose. As the air descends into the lungs where it gets humidified, the concentration of oxygen decreases. At the level of the trachea of the lungs it is 149 mm Hg. At the alveoli, where gas exchange occurs, the oxygen concentration is 105 mm Hg because of the higher humidity at that level. When the atmospheric air is humid, there is even less oxygen in it and therefore less oxygen that enters the mouth and nose. As that air descends to the alveoli, the oxygen content is much lower compared to the non-humid air example.
A person with a PFO has lower blood oxygen saturation in humid weather. And if it is hot and humid, that person has a much harder time breathing and fulfilling the body’s needs for oxygen.
HIGH ALTITUDE
High altitude reduces breathing response, decreases the ability to oxygenate the blood and increases the susceptibility to Acute Mountain Sickness (AMS). Altitude sickness can begin at 2000 – 2500 meters, but some with chronic medical conditions are at risk at even lower altitudes. Rapid ascent decreases the time for acclimatization and hence is the primary reason for acute altitude illness.
A study (14) done on 21 healthy 20-year-old subjects (11 with relatively small PFO) at a research facility at Mt. Chacaltaya, Bolivia, 5,260 m = 17,257 feet showed that gas exchange got worse for all people with or without a PFO when they were taken directly up to this high altitude. The oxygen saturation was about the same for those without or with a PFO: 67% vs 66%, respectively. For people without a PFO the oxygen saturation improved to 76% after 16 days of acclimatization because more oxygen is taken in by the lungs (increased alveolar ventilation).
But people with a PFO did not attain acclimatization after 16 days – both at rest or while exercising – and were still suffering from Acute Mountain Sickness (oxygen saturation = 70%). Symptoms of Acute Mountain Sickness include headache, anorexia, insomnia, breathlessness, and unsteady gait using the Lake Louise scoring system (15). They also had a higher risk for high altitude pulmonary edema (fluid in lung), stroke, heart attack, and damage to other organs.
One way to partially compensate for a decreased intake of oxygen at high altitudes is to slow down your breathing rate but increase the depth of your breaths and inhale until your abdomen expands.
People who are born and live at high altitude have the same prevalence and sizes of PFOs as those who are low-altitude natives. Apparently, selective adaptation did not eliminate PFOs for those living at high altitude. Tibetans, who thrive at altitudes over 4400 meters (14,435 feet) and whose ancestors have lived at high altitudes for thousands of years, have two gene variants (EGLN1 and PPARA) that help them use oxygen more efficiently with less hemoglobin than people who live at low altitudes – a process that may involve nitric oxide (16).
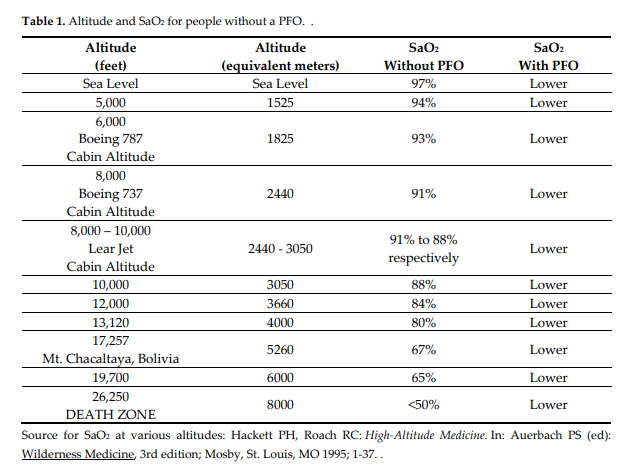
People with a PFO traveling in a Learjet (cabin altitude 8,000-10,000 feet), Boeing 737 (cabin altitude 8,000 feet), or a Boeing 787 (cabin altitude 6,000 feet), will have an SaO2 lower than 88%-91%, 91%, 93% respectively, and below 95% is considered low.
AIR FLIGHT
US Federal Aviation Regulations state that pressurized cabins must provide a cabin altitude of not more than 8000 feet at the maximum operating altitude of the airplane (FAA, 1996), so that the partial pressure of oxygen be maintained at 0.17–0.15 at sea level. Cabin altitude measurements by 28 airlines range from sea level to 8,915 feet.
The Boeing 737’s cabin altitude can climb to 8,000 feet while the aircraft’s actual altitude is 41,000. Newer aircraft such as the Boeing 787 are designed to have lower cabin altitudes of 6,000 feet. At 35,000 feet in a Learjet, the cabin altitude may be 8,000 to a maximum of 10,000 feet. There is less oxygen in the air at higher altitudes and our ability to get enough oxygen decreases. Alveolar oxygen tension decreases to 65 mm Hg at 8000 feet that reduces arterial oxygen tension to 60 mm Hg in healthy individuals (17). Patients with chronic hypoxia may have a greater reduction in SaO2 when they reach high altitude than those with healthy lungs. Any small reduction in the oxygen content of the air in the cabin results in a significant decrease in the SaO2 because of the oxyhemoglobin dissociation curve. This is even worse for those with PFOs.
In a study of 84 passengers aged 1–78 years not tested for PFO, researchers recorded their SaO2s by pulse oximetry at sea level and during air travel on 10 different airlines at altitudes ranging from 27,000 to 37,000 feet (18). There was a significant reduction in oxygen saturation in all passengers travelling long haul and short haul flights that lasted 1 hour or more. The mean SaO2 for all flights at ground level was 97% [93% to 100% +/- 1.33] and at cruising altitude 93% [85% to 98% +/-2.33]. Fifty-four per cent of passengers had SaO2 of 94% or less at cruising altitude for which more than one-third of respiratory physicians would prescribe supplementary oxygen (19). No individual had severe cardio-pulmonary problems, and no one required permission from their doctor to fly. Their smoking status was unknown.
Low SaO2 (hypoxia) increases the risk for DVT (Deep Venous Thrombosis) because it increases coagulation, dilates blood vessels and increases capillary permeability. These blood clots in the legs can break off and go to the lungs in a person without a PFO or can go to the brain, heart, or any other organ in a person with a PFO. Other risk factors for causing DVT are reduced blood flow (immobility in the aircraft), dehydration, and damage to blood vessels.
ALTITUDE DECOMPRESSION SICKNESS
Altitude Decompression Sickness is caused by venous gas emboli that cross a PFO or other potential right to left shunt, move to the arterial side and cause stroke-like symptoms or heart attack. This results from exposure to low barometric pressures that cause inert gases (mainly nitrogen), normally dissolved in body fluids and tissues, to come out of solution in bubbles just like opening a carbonated soft drink – the liquid goes from high pressure to a lower barometric pressure, gas is heard escaping, and bubbles can be seen forming. The venous gas emboli can affect:
1) LARGE JOINTS – BENDS 60%-70%: pain can occur at altitude, during descent, or hours later.
2) NEUROLOGIC 10%-15%
BRAIN: Confusion or memory loss • Headache • Spots in visual field (scotoma), tunnel vision, double vision (diplopia), or blurry vision • Behavior changes • Seizures, dizziness, vertigo, nausea, vomiting and unconsciousness
SPINAL CORD: Burning, stinging, tingling around the lower chest and back, abdominal or chest pain • Ascending weakness or paralysis.
PERIPHERAL NERVES: Urinary and rectal incontinence • Numbness, burning, stinging and tingling • Muscle weakness or twitching
3) PULMONARY DECOMPRESSION SICKNESS is a rare but severe form of decompression sickness that can be rapidly fatal if not treated. • Burning deep chest pain (under the sternum) • Pain is aggravated by breathing • Shortness of breath
4) SKIN BENDS: Itching around the ears, face, neck, arms, and upper torso • Sensation of tiny insects crawling over the skin
Individual susceptibility can vary from day to day, and different individuals under the same conditions may be affected differently or not at all. Altitude Decompression Sickness, whether associated with PFO, is well studied in the military literature and has been a particular hazard for pilots operating very high-altitude aircraft, such as the U-2 (20).
Table 2. Risk Factors For Developing Altitude Decompression Sickness
1) Altitude higher than 18,000 feet
2) Flying an unpressurized aircraft to altitude
3) Fast rate of ascent
4) Mountain climbing
5) Older age
6) Overweight – nitrogen is stored in greater amounts in fat
7) Alcohol
8) Scuba Diving before flying – a person breaths air under high pressure that increases the amount of nitrogen dissolved in the body
PLATYPNEA-ORTHODEOXIA is a rare clinical syndrome characterized by shortness of breath and deoxygenation when a person with a PFO or other atrial septal defect sits or stands from a lying down position thus stretching the foramen allowing more streaming of venous blood especially when there is a persistent Eustachian valve (21). There are four causes of platypnea-orthodeoxia syndrome: intracardiac shunting, pulmonary shunting, ventilation-perfusion mismatch, or a combination of these. Closure of the foramen is the treatment.
ELECTROCARDIOGRAM (ECG) PATTERNS SEEN IN PFO PATIENTS
Even before echocardiography confirmation, ECG patterns can help diagnose congenital interatrial septal disorders (ASD) found in adults that include PFO, atrial septal defect, and atrial septal aneurysm. More than clinical signs and symptoms, right bundle branch block (RBBB) is the most frequent conduction disorder found in 77% of patients with ASD (22, 23).
The crochetage pattern is a useful ECG sign to predict ASD (24, 25). The crochetage pattern, a notch near the apex of the R-wave in inferior limb lead, correlates with the degree of left-to-right shunt and with the size of the ASD. The specificity is 92.6% and sensitivity is 73.1% with a positive predictive value of 69% if the crochetage pattern is present in one lead, and the specificity becomes 100% if the sign is present in all three inferior leads. An ECG diagnosis of ASD can be made if the crochetage pattern is present in all inferior limb leads together with RBBB.
Patients with PFO and crochetage pattern are more likely to have cerebral infarction than non-PFO patients with crochetage (26). The crochetage pattern can recognize PFO patients who are prone to paradoxical embolism with a specificity of 91% and a positive predictive value of 77%. With surgical correction of the ASD, the crochetage pattern disappeared but the RBBB did not.
Another ECG pattern to predict ASD is the defective T-wave defined as “inverted or horizontal displacement of the proximal T-wave limb in the right precordial leads.” The diagnosis of ASD can be made if there is an incomplete RBBB and a defective T-wave: 100% specificity and 87.1% sensitivity (27).
CURRENT TREATMENT OF PATIENTS WITH PFO
Prior to randomized trials, closure of the patent foramen ovale for those who had a stroke was done even though there was no definitive evidence that closure prevented a subsequent stroke.
CLOSURE I [Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale] was the first large, randomized trial (909 patients) and showed no difference for preventing recurrent ischemic stroke for 462 patients who received medical therapy compared to 447 patients who had PFO closure with the STARFlex device (28).
RESPECT 2013, the second randomized trial involving 980 patients [Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment]: 499 patients had PFO closure with the Amplatzer PFO Occluder and 481 assigned to medical management (29). However, most patients in both groups had antiplatelet treatment. There was no difference between the two treatment arms for preventing recurrent ischemic stroke.
PC Trial [Percutaneous closure of patent foramen ovale in cryptogenic embolism] was the third randomized trial involving 414 patients in 29 centers in Europe, Canada, Brazil and Australia (30). The Amplatzer PFO Occluder was installed in 204 patients while 210 patients had medical therapy. Again, there was no difference between the two treatment arms for preventing recurrent ischemic stroke.
The newer trials suggest PFO closure:
CLOSE [Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke]. 663 patients: 238 patients assigned to closure using a device approved by Interventional Cardiology Committee + dual antiplatelet treatment (75 mg aspirin + 75 mg clopidogrel; 235 patients assigned to anticoagulants, or aspirin, or clopidogrel, or aspirin + extended-release dipyridamole) (31).
RESPECT 2017 [Long-term outcomes of patent foramen ovale closure or medical therapy after stroke]. 980 patients: 3141 patient-years in the closure group (Amplatzer PFO Occluder + 81 to 325 mg aspirin plus clopidogrel for 1 month, then aspirin alone for 5 months, then anticoagulation as per physician; 2669 patient-years in the medical treatment group (aspirin, or warfarin, or clopidogrel, or aspirin + extended-release dipyridamole) (32).
REDUCE [Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke]. 664 patients: 441 patients assigned to closure using Helex Septal Occluder or Cardioform Septal Occluder both by W.L. Gore and Associates + antiplatelet therapy; 223 patients assigned to antiplatelet therapy. Antiplatelet therapy consisted of 75 to 325 mg aspirin, or a combination of aspirin 50 to 100 mg + 225 to 400 mg dipyridamole, or 75 mg clopidogrel (33).
However, each of these trials had limitations:
(1) There was no consistent dose of aspirin, antiplatelet or anticoagulant within the trials. No trial identified how many people took what aspirin dose (ranged from 50 mg to 325 mg), enteric coated or not, and at what time they took it. Uncoated aspirin 325 mg at 6 PM decreases risk for heart attack, stroke, cancer, colon cancer, colon polyps, and decreases lipoprotein(a).
There was no indication of aspirin adherence that can cause aspirin treatment failure (34). And there was no indication of patients taking non-steroidal anti-inflammatory drugs that can impair aspirin effectiveness and lead to aspirin treatment failure (28, 29, 35).
Only the CLOSE (31) trial indicated Body Mass Index >30 but did not indicate the dose of aspirin for those patients which is very important because “low dose aspirin (75-100 mg/day) is only effective in preventing vascular events in patients weighing less than 154 lb (70 kg) and had no benefit in 80% of men and 50% of women weighing 154 lb (70 kg) or more” (36).
There was no measurement of increased platelet turnover commonly seen in diabetics that can lead to aspirin treatment failure (37). The percentage of diabetics was higher in the antiplatelet only treatment groups (31, 33) and medical therapy only group (32) compared to the PFO closure groups (31-33).
In addition to its antiplatelet effect, 325 mg aspirin inhibits thromboxane A2 much more rapidly than 81 mg and therefore acts more quickly as an anticoagulant.
Aspirin is rapidly absorbed with a bioavailability of about 50%, but much lower when given as the enteric coated form (38, 39). Peak concentration of non-coated aspirin occurs rapidly within 30 minutes after ingestion but up to 4 hours after ingesting enteric coated aspirin (40).
The fibrinolytic system has a circadian rhythm peaking at 6 PM with its lowest level of activity at 6 AM. We are unprotected in the early hours of the morning – in fact, most heart attacks and strokes occur between 3 AM and 5 AM. Therefore, take a non-coated 325 mg aspirin at around 6 PM with your large meal so that you have protection throughout the night.
(2) The REDUCE trial showed the same number of silent brain infarcts with or without closure (33).
(3) Complete closure of the PFO in the REDUCE trial was attained by only 75% of patients at the end of 12 months (33).
(4) W.L. Gore and Associates, the sponsor of the REDUCE trial, “was responsible for data management and provided the statistician. The sponsor assisted in the creation of the figures and was given the opportunity to review the manuscript.” (33). All authors report Personal Fees, and/or Grants, and/or “Other” from W.L. Gore and Associates.
Because of these PFO cryptogenic stroke studies, many people may continue aspirin treatment only because: (1) there is a low risk of recurrent stroke with no device; (2) there is doubt of how much benefit the device actually provides; and, (3) there is a risk of complications at the time of device implantation and from having a device in your heart (arrhythmias, clots from the device, etc.) [41, 42]. The aspirin they choose may be suboptimal for dose, coating on the aspirin, and time they take it. None of these trials, however, considered the important hypoxia physiological consequences described above.
PHYSICIANS SHOULD SUSPECT A PFO IF A PATIENT HAS:
Unexplained stroke, acute myocardial infarction, acute partial blindness, loss of consciousness, migraines with aura, acute kidney dysfunction, or any other acute organ dysfunction (hypoxia, hypercoagulable state, unfiltered clots, unfiltered air bubbles, unfiltered fat emboli [Altitude Decompression Sickness])
Unexplained chest pain, confusion, headaches, rapid heartbeat
Unexplained hypercoagulable state
Unexplained / unusual shortness of breath at high altitude that increases the susceptibility to Acute Mountain Sickness
Learjet SaO2 91%-88% – in a person without PFO, lower for a person with a PFO
Boeing 737 SaO2 91% – in a person without PFO, lower for a person with a PFO
Boeing 787 SaO2 93% – in a person without PFO, lower for a person with a PFO
Unexplained worse/profound jet lag
Unexplained overheating or feeling completely “drained and exhausted” within a few minutes of being in the heat/humidity, accompanied by shortness of breath with normal blood pressure – higher core temperature at rest and during exercise resulting in an even lower oxygen level. A person with a PFO will have a lower SaO2 in humid weather. If it is hot and humid, the person with a PFO has a much more difficult time breathing and fulfilling the body’s oxygen needs.
Unexplained worsening of an outpatient’s condition being treated for pneumonia
Unexplained signs and symptoms that are related to Altitude Decompression Sickness: Bends, Neurologic (brain, spinal cord, peripheral nerves), Chokes (lung), skin Bends
Unexplained worsening of symptoms with aging
CONCLUSION
This information, some of which is only recently available, presented succinctly and comprehensively for the first time, will provoke thoughtful discussion between physicians and patients with PFOs particularly for those whose PFOs get larger with aging. A suggestion can be made to close the foramen if there are no medical contraindications for closure.
REFERENCES
-
Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. Jan 59(1): 17-20. 1984. DOI: 10.1016/s0025-6196(12)60336-x
-
Woods TD, Harmann L, Purath T, Ramamurthy S, Subramanian S, Jackson S, Tarima S. Small and moderate size right-to-left shunts identified by saline contrast echocardiography are normal and unrelated to migraine headache. Chest.138: 264-269; 2010. 10.1378/chest.09-2797
-
Elliott JE, Nigam SM, Laurie SS, Beasley KM, Goodman RD, Hawn JA, Gladstone IM, Chestnutt MS, Lovering AT. Prevalence of left heart contrast in healthy young asymptomatic humans at rest breathing room air. Respir Physiol Neurobiol. 188: 71-78; 2013. 10.1016/j.resp.2013.04.019
-
Marriott K, Manins V, Forshaw A, Wright J, Pascoe R. Detection of right-to-left atrial communication using agitated saline contrast imaging experience with 1,162 patients and recommendations for echocardiography. J. Am. Soc. Electrocardiogr. 26: 96-102. 2013. https://doi.org/10.1016/j.echo.2012.09.007
-
Rivadeneira JVA, Romo H. Diagnosis of patent foramen Ovale: Concordance between transthoracic and transesophageal echocardiography using agitated saline. Rev Argent Cardiol. 84:472-473. 2016. 10.7775/RAC.84.5.7611
-
Bang OY, Lee MJ, Ryoo S, Kim SJ, Kim JW. Patent Foramen Ovale and Stroke – Current Status. J Stroke. Sep; 17(3):229-237. 2015. 10.5853/jos.2015.17.3.229
-
Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. Oct 24; 55(8):1172-9. 2000. 10.1212/wnl.55.8.1172
-
Homma S, Sacco RL. Patent Foramen Ovale and Stroke. Circulation.112:1063–1072. 2005. 10.1161/01.STR.0000127038.10584.27
-
Lovering A, Elliott JE, Davis JT. Physiological impact of patent foramen ovale on pulmonary gas exchange, ventilatory acclimatization, and thermoregulation. J Appl Physiol121: 512–517, 2016. 10.1152/japplphysiol.00192.2015
-
Lovering A, Lozo M, Barak O, Davis JT, Lojpur M, Lozo P, Caljkusic K and Zeljko Dujic. Resting arterial hypoxemia in subjects with chronic heart failure, pulmonary hypertension and patent foramen ovale. Exp Physiol. 101. 5:657-70. 2016. 10.1113/EP085657
-
Fenster BE, Nguyen BH, Buckner JK, Freeman AM, Carroll JD. Effectiveness of percutaneous closure of patent foramen ovale for hypoxemia. Am J Cardiol112: 1258–1262, 2013. 10.1016/j.amjcard.2013.06.022
-
Bendz B, Rostrup M, Sevre K, Andersen TO, Sandset PM. Association between acute hypobaric hypoxia and activation of coagulation in human beings. Lancet; 356: 9242; 2000. 10.1016/S0140-6736(00)03165-2
-
Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia. Clinical Infectious Diseases, Volume 52(3), 1 February 2011, Pages 325–331. 10.1093/cid/ciq076
-
Elliott JE, Laurie SS, Kern JP, Beasley KM, Goodman RD, Kayser B, Subudhi AW, Roach RC, Lovering AT. AltitudeOmics: impaired pulmonary gas exchange efficiency and blunted acclimatization in humans with patent foramen ovale after 16 days at 5,260 m. J Appl Physiol118: 1100–1112, 2015. 10.1152/japplphysiol.00879.2014
-
Roach RC, Hackett PH, Oelz O et al. The 2018 Lake Louise Acute Mountain Sickness Score. High Altitude Med & Biology. 2018. 19(1): 4-6. 10.1089/ham.2017.0164
-
Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science. 329(5987): 72-75. July 2010. 10.1126/science.1189406
-
Cottrell JJ. Altitude exposures during aircraft flight: flying higher. Chest.92: 81–4. 1992. 10.1378/chest.93.1.81
-
Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anesthesia. 2005. 60:458-460. 10.1111/j.1365-2044.2005.04124.x
-
Coker RK, Partridge MR. Assessing the risk of hypoxia in flight: the need for more rational guidelines. European Respiratory Journal.15: 128–30; 2000. 10.1183/09031936.00.15112800
-
-
Henkin S, Negrotto S, Pollak P, et al. Platypnea-Orthodeoxia Syndrome: Diagnostic Challenge and the Importance of Heightened Clinical Suspicion. Tex Heart Inst J. 2015 Oct; 42(5): 498-501. 10.14503/THIJ-14-4596
-
Bakalli A, Pllana E, Kocinaj D, Bekteshi T, Dragusha G, Gashi M, Musliu N, Gashi Z. Association of interatrial septal abnormalities with cardiac impulse conduction disorders in adult patients: experience from a tertiary center in Kosovo. Heart Int. 2011 Jun 2; 6(1):e4. Published online 2011 Jul 21. 10.4081/hi.2011.e4
-
Bakalli A, Kocinaj D, Georgievska-Ismail L, Bekteshi T, Pllana E, Seidiu B. Right Bundle Branch Block as a Marker for Interatrial Septal Abnormalities. Cardiol Young. 2012 Feb;22(1):18-25. 10.1017/S1047951111000795
-
Heller J, Hagège AA, Besse B, Desnos M, Marie FN, Guerot C. “Crochetage” (notch) on R wave in inferior limb leads: A new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877–82. 10.1016/0735-1097(95)00554-4
-
Raut MS, Verma A, Maheshwari A, Shivnani G. Think Beyond Right Bundle Branch Block in Atrial Septal Defect. Ann Card Anaesth. 2017 Oct-Dec; 20(4): 475–476. 10.4103/aca.ACA_5_17
-
Ay H, Buonanno FS, Abraham SA, Kistler JP, Koroshetz WJ. An electrocardiographic criterion for diagnosis of patent foramen ovale associated with ischemic stroke. Stroke. 1998;29:1393–7. 10.1161/01.str.29.7.1393
-
Wang MX, Wu GF, Gu JL, Li L, Lu K, Yang D, et al. Defective T wave combined with incomplete right bundle branch block: A new electrocardiographic index for diagnosing atrial septal defect. Chin Med J (Engl) 2012;125:1057–62. https://pubmed.ncbi.nlm.nih.gov/22613531/
-
Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. CLOSURE I. N Engl J Med2012; 366:991-999March 15, 2012DOI: 10.1056/NEJMoa1009639
-
Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. RESPECT N Engl J Med2013; 368:1092-1100 March 21, 2013DOI: 10.1056/NEJMoa1301440
-
Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P. Percutaneous closure of patent foramen ovale in cryptogenic embolism. PC Trial. N Engl J Med2013; 368:1083-1091March 21, 2013DOI: 10.1056/NEJMoa1211716
-
Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. CLOSE. N Engl J Med2017; 377:1011-1021 September 14, 2017DOI: 10.1056/NEJMoa1705915
-
Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, and Tirschwell DL. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. RESPECT (2017). N Engl J Med2017; 377:1022-1032September 14, 2017DOI: 10.1056/NEJMoa1610057
-
Sondergaard, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. REDUCE, N Engl J Med2017; 377:1033-1042September 14, 2017DOI: 10.1056/NEJMoa1707404.
-
Hennekens CH, Schneider WR, Herbert PR, Tantry US, Gurbel, PA. Hypothesis formulation from subgroup analyses: nonadherence or nonsteroidal anti-inflammatory drug use explains the lack of clinical benefit of aspirin on first myocardial infarction attributed to “aspirin resistance.” Am Heart J. 2010 May;159(5):744-8 10.1016/j.ahj.2009.11.033
-
Li X, Fries S, Li R, Lawson JA, Propert KJ, Diamond SL, Blair IA, FitzGerald GA, Grosser T. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA. 2014 Nov 25;111(47):16830-5. 10.1073/pnas.1406997111
-
Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncagliani MC, Morimoto T, Mehta Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomized trials. Lancet. 2018; 392:387-99. 10.1016/S0140-6736(18)31133-4.
-
Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, Pocaterra D, Ragazzoni E, Rolandi G, Rocca B, Patrono C. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood. 2012 Apr 12;119(15):3595-603. 10.1182/blood-2011-06-359224
-
Maree AO, Curtin RJ, Dooley M, et al. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol. 2005; 46:1258-63. 10.1016/j.jacc.2005.06.058
-
Grosser T, Fries S, Lawson JA, Kapoor SC, Grant Gr, FitzGerald GA. Drug resistance and pseudoresistance: an unintended consequence of enteric coating aspirin. Circulation. 2013; 127:377-85. 10.1161/CIRCULATIONAHA.112.117283
-
Patrono C. Aspirin. Chapter 53. Platelets. Academic Press. 2013. Pages 1099-1115. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.15966
-
Abaci A, Unlu S, Alsancak Y, Sezenoz B. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: Meta-analysis of 28,142 patients from 203 studies. Cathet Cardiovasc Intervent. 82: 1123–1138. 2013. 10.1002/ccd.24875
-
Mirzada N, Ladenvall P, Hansson PO. Seven-year follow-up of percutaneous closure of patent foramen ovale. IJC Heart and Vasculature. 1:32-36. 2013. 10.1016/j.ijchv.2013.11.003
(c) 2010, 2024 Charles B. Simone, M.MS., M.D.