We do not diagnose disease or recommend a dietary supplement for the treatment of disease. You should share this information with your physician who can determine what nutrition, disease and injury treatment regimen is best for you. You can search this site or the web for topics of interest that I may have written (use Dr Simone and topic).
“We provide truthful information without emotion or influence from the medical establishment, pharmaceutical industry, national organizations, special interest groups or government agencies.” Charles B Simone, M.MS., M.D.
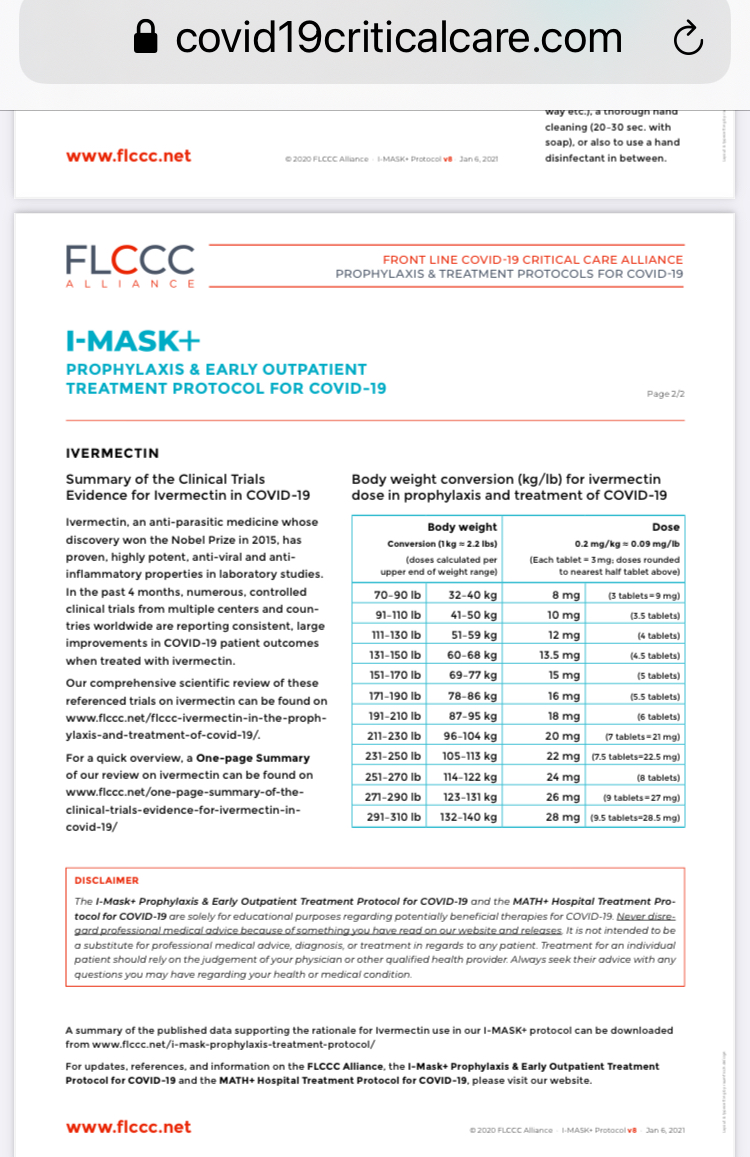
IVERMECTIN SAVES LIVES
https://bit.ly/3aCNAtl
Lawrenceville, NJ (Charles B Simone, M.MS., M.D.) – There are 81 separate studies — involving a combined 128,000 participants — that demonstrated an average efficacy of 65% for several different outcomes. There are also 22 studies — involving nearly 40,000 people — around the outcome in question, hospitalization. Those studies showed an average efficacy of 39%.
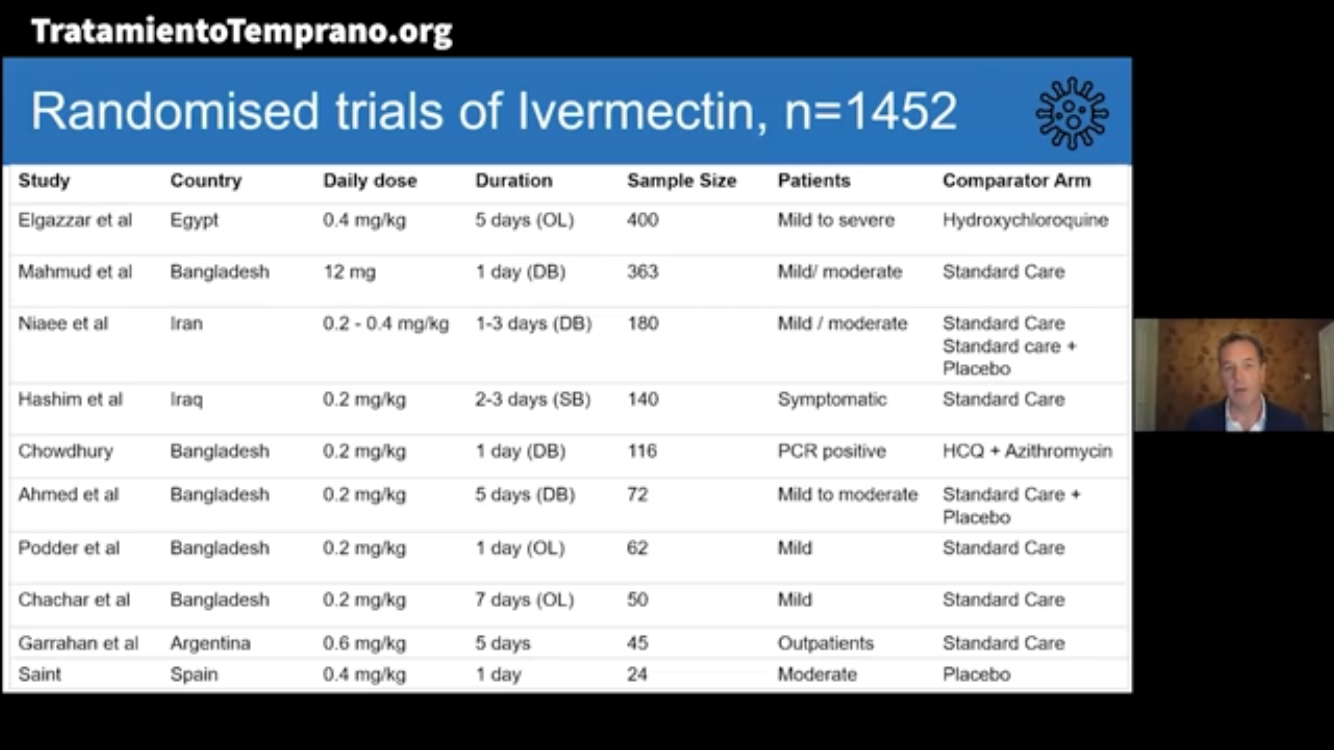
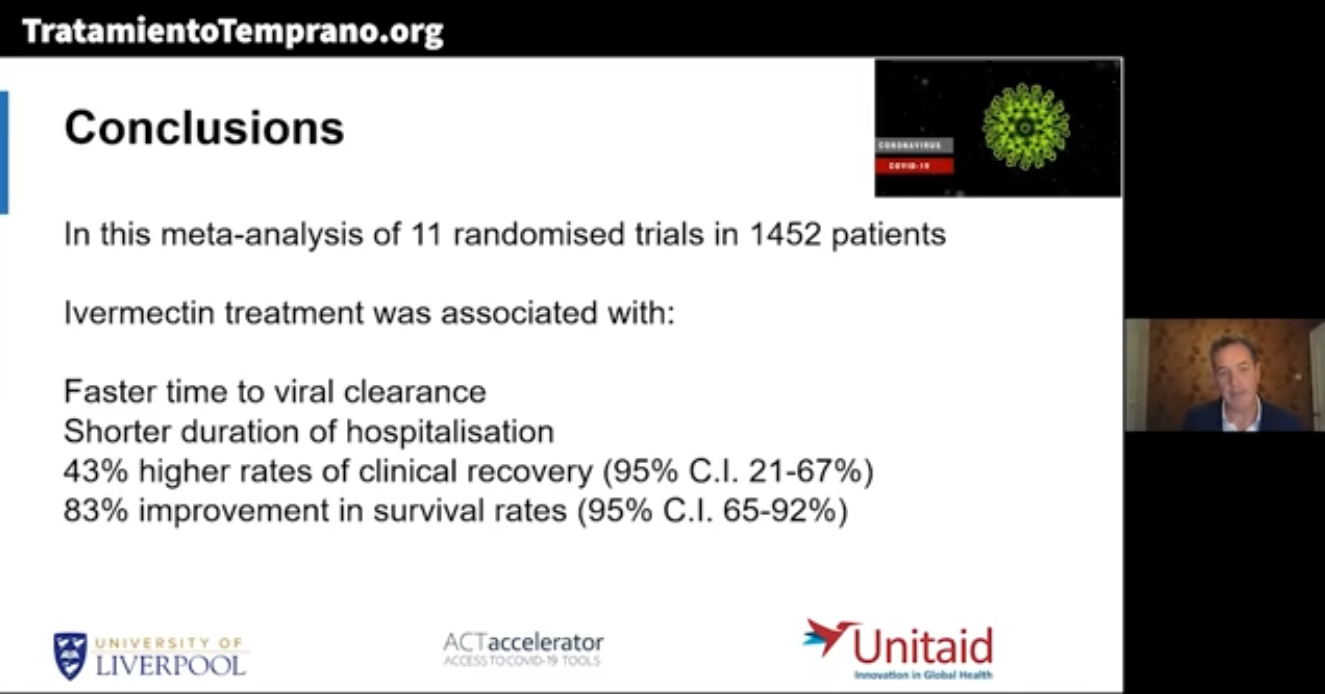
The World Health Organization – Swiss Policy Research – sponsored a review of 11 randomized trials involving 1456 patients that shows ivermectin, a safe prescription drug, saves lives – an 83% reduction in dying from COVID-19. There are 45 more trials ongoing with 7100 patients.
Ivermectin also has anticancer effects.
Meta-analyses based on 18 randomized controlled treatment trials of ivermectin in COVID-19 have found large, statistically significant reductions in mortality, time to clinical recovery, and time to viral clearance.
In summary, based on the totality of the trials and epidemiologic evidence presented in this review along with the preliminary findings of the Unitaid/WHO meta-analysis of treatment RCTs and the guideline recommendation from the international BIRD conference, ivermectin should be globally and systematically deployed in the prevention and treatment of COVID-19.

Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19.
https://pdfs.journals.lww.com/americantherapeutics/2021/06000/Review_of_the_Emerging_Evidence_Demonstrating_the.4.pdf?token=method|ExpireAbsolute;source|Journals;ttl|1624307490952;payload|mY8D3u1TCCsNvP5E421JYK6N6XICDamxByyYpaNzk7FKjTaa1Yz22MivkHZqjGP4kdS2v0J76WGAnHACH69s21Csk0OpQi3YbjEMdSoz2UhVybFqQxA7lKwSUlA502zQZr96TQRwhVlocEp/sJ586aVbcBFlltKNKo+tbuMfL73hiPqJliudqs17cHeLcLbV/CqjlP3IO0jGHlHQtJWcICDdAyGJMnpi6RlbEJaRheGeh5z5uvqz3FLHgPKVXJzdZJ8ccq6X4sEZ2NPLvMHQDuxDWf7uJaSPnBJLTN9vhZHiBU4oRQjZFIWyeQz+S9i6;hash|wNUVu59IUa7/qsUFMToNBw==
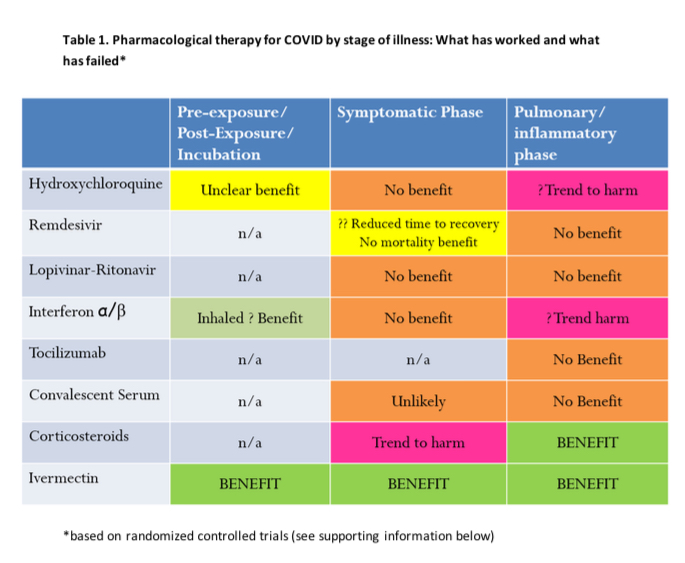
Ivermectin and Hydroxychloroquine are lysosomotropic agents that should be used within the first five days of exposure to SARS-CoV 2 (COVID-19). These medicines cross the lysosomal membrane and become protonated thus increasing the lysosome’s pH (alkaline) rendering the lysosome nonfunctional. These medicines include Hydroxychloroquine and Ivermectin.
HYDROXYCHLOROQUINE SAVES LIVES https://bit.ly/38t2ZKl
Ivermectin for Prevention and Treatment of COVID-19 Infection:
A Systematic Review, Meta-analysis, and Trial
Sequential Analysis to Inform Clinical Guidelines
In this meta-analysis of 15 trials ivermectin reduced the risk of death compared with no ivermectin. Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin.
Ivermectin prophylaxis reduced COVID-19 infection by an average 86%
Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.
This result was confirmed in a trial sequential analysis using the
same DerSimonian–Laird method that underpinned the unadjusted analysis. This was also robust against a trial sequential analysis using the Biggerstaff–Tweedie method.

Ivermectin for Prevention and Treatment of COVID-19 Infection. Bryant et al. American Journal of Therapeutics: June 17, 2021 – Volume Publish Ahead of Print – Issue – doi: 10.1097/MJT.0000000000001402
file:///C:/Users/chuck/Downloads/Ivermectin_for_Prevention_and_Treatment_of.98040.pdf
In another study published January 15, 2022 done in Itajaí, Santa Catarina, Brazil regular use of ivermectin as a prophylactic agent was associated with significantly reduced COVID-19 infection, hospitalization, and mortality rates.
Of the 223,128 citizens of Itajaí considered for the study, a total of 159,561 subjects were included in the analysis: 113,845 (71.3%) regular ivermectin users and 45,716 (23.3%) non-users. Of these, 4,311 ivermectin users were infected, among which 4,197 were from the city of Itajaí (3.7% infection rate), and 3,034 non-users (from Itajaí) were infected (6.6% infection rate), with a 44% reduction in COVID-19 infection rate (risk ratio [RR], 0.56; 95% confidence interval (95% CI), 0.53-0.58; p < 0.0001). Using PSM, two cohorts of 3,034 subjects suffering from COVID-19 infection were compared. The regular use of ivermectin led to a 68% reduction in COVID-19 mortality (25 [0.8%] versus 79 [2.6%] among ivermectin non-users; RR, 0.32; 95% CI, 0.20-0.49; p < 0.0001). When adjusted for residual variables, reduction in mortality rate was 70% (RR, 0.30; 95% CI, 0.19-0.46; p < 0.0001). There was a 56% reduction in hospitalization rate (44 versus 99 hospitalizations among ivermectin users and non-users, respectively; RR, 0.44; 95% CI, 0.31-0.63; p < 0.0001). After adjustment for residual variables, reduction in hospitalization rate was 67% (RR, 0.33; 95% CI, 023-0.66; p < 0.0001).
The Egyptian trial showed the strongest treatment effects: 5 days of 0.4 mg/day (28 mg per day for a 70 kg person 154 lb) compared to the Bangladesh trial of 1 day of 0.2 mg/day (14 mg per day for a 70 kg person 154 lb). Cost in U.S is $75 for 50 tablets each containing 3 mg; and far less costly in Bangladesh.
Ivermectin is an anti-parasite medicine used for more than 40 years. It is on the WHO’s list of essential medicines, has been given 3.7 billion times around the globe, and won the Nobel prize for its global and historic impacts in eradicating human endemic parasitic infections. Ivermectin inhibits SARS-CoV-2 replication and suppresses inflammation. It also seems to be safe for people with Myasthenia Gravis, whereas Hydroxychloroquine is not.
https://swprs.org/who-preliminary-review-confirms-ivermectin-effectiveness/
Dr. Pierre Kory testifies to Senate Committee about Ivermectin, Dec. 8, 2020
https://covid19criticalcare.com
Preprint Manuscript https://osf.io/wx3zn/

https://www.evms.edu/media/evms_public/departments/internal_medicine/EVMS_Critical_Care_COVID-19_Protocol.pdf?fbclid=IwAR20F7G10ICCiZVXuoPJMfluNNMlUbnDyKsfpkvdvZ1XX2IngpBGDKg4Nxg
WHO SUPPRESSED IVERMECTIN’s USE
1) MEDIA –
New York Times – “Senate hearing promoted unproven drugs and dubious claims about ivermectin.”
AP – “No evidence ivermectin is a miracle drug against COVID-19”
2) BIG TECH –
YouTube, owned by Google – banned ivermectin information from Dr Risinger and Dr. Martenson.
Twitter – blacklisted European Medical Journal for published study on ivermectin.
Facebook – removed posts of Front Line Covid-19 Critical Care
3) NIH – formed a panel of doctors who decided what treatments can be given to patients. Ivermectin was not on the list and, in fact, the panel recommended use Remdesivir as highly recommended by Fauci – “diminishes the time to recovery.” Midway through the study, the endpoint was changed from mortality to recovery because Remdesivir does not save lives. The World Health Organization does not recommend it at all. Gilead owns Remdesivir. Seven members of the panel are paid by Gilead, including the three co-chairs of the panel who choose the panel members.
August 8, 2020 – India Times – Ivermectin to be used for Covid treatment in Uttar Pradesh (a state in India with 230 million people) as a new medication for the treatment and prevention of Covid-19. In January 2021, fewer than 10 deaths a days while in the U.S. there were more than 3,000 deaths per day.
EL SALVADOR GIVES COVID PATIENTS CARE PACKAGE OF THERAPEUTIC DRUGS
El Salvador government gives this package that includes ivermectin to its citizens with COVID-19 or those who are exposed to people with COVID-19. Video is from BitChute.
To obtain Ivermectin call your own physician or one listed by Front Line COVID-19 Critical Care Alliance at https://covid19criticalcare.com/ivermectin-in-covid-19/how-to-get-ivermectin/
The following list of pharmacies will fill prescriptions for ivermectin https://covid19criticalcare.com/pharmacies/
WSJ Misleads Public on Ivermectin, Ignores Latest Revelations About ‘Hidden Author’ Who Undermined Its Efficacy
When Lawrie confronted a squirming Hill, Hill eventually admitted the conclusions in his analysis had been influenced by Unitaid, a quasi-governmental advocacy organization funded by the Bill & Melinda Gates Foundation. Unitaid gave $40 million to Andrew Hill’s employer, the University of Liverpool, four days before the publication of Hill’s study. Hill confessed that the sponsors were pressuring him to influence his conclusion.
IVERMECTIN ALSO HAS ANTICANCER EFFECTS
Ivermectin has powerful antitumor effects, including the inhibition of proliferation, metastasis, and angiogenic activity, in a variety of cancer cells. This may be related to the regulation of multiple signaling pathways by ivermectin through PAK1 kinase. On the other hand, ivermectin promotes programmed cancer cell death, including apoptosis, autophagy and pyroptosis. Ivermectin induces apoptosis and autophagy is mutually regulated. Interestingly, ivermectin can also inhibit tumor stem cells and reverse multidrug resistance and exerts the optimal effect when used in combination with other chemotherapy drugs.

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7505114/pdf/main.pdf
IVERMECTIN and Multiple Sclerosis – Anti-inflammatory action, neuroprotection and re-myelination!
From Dr Makis
2023 Noori et al – Synthesis and evaluation of the effects of solid lipid nanoparticles of ivermectin and ivermectin on cuprizone-induced demyelination via targeting the TRPA1/NF-kB/GFAP signaling pathway
“Ivermectin (IVM) and nano-IVM administration improved behavioral alterations and motor balance in all performed tests demonstrating a reduction in the adverse effects of Cuprizone (CPZ) on myelin”
“To study MS in animal models, the most common method is to trigger the pathology with cuprizone (CPZ). CPZ is a copper chelator whose use induces programmed death in oligodendrocyte cells, chronic inflammation, increased astrocyte and microglial cell activity, and ultimately, myelin destruction“
“Histological analysis (H&E and LFB staining) evidenced that IVM and nano-IVM normalized the morphological alteration induced by CPZ”
“Specifically, the nano-formulation of IVM improved its neuroprotective effects via reducing demyelination or maybe inducing myelin regeneration.”
This adds to the 2018 study by Zabala et al: “Our results provide evidence that P2X4Rs modulate microglia/macrophage inflammatory responses and identify IVM as a potential candidate among currently used drugs to promote the repair of myelin damage.“
“Manipulating innate immune system to promote repair might be a promising therapeutic strategy for treating MS. The results of our study identify P2X4R as a key modulator of microglia/macrophage polarization and support the use of IVM to potentiate a microglia/macrophage switch that favors remyelination in MS.”
“The fact that IVM is already used as an anti‐parasitic agent in humans will facilitate challenging this drug in clinical trials in that demyelinating disease.”
emails to Dr Makis about Ivermectin: 80% asked about cancer, 10% asked about parasites, 8% asked about Long COVID, 1-2% asked about Multiple Sclerosis, ALS, Alzheimer’s Dementia
Pfizer and Moderna COVID-19 mRNA Vaccines cause several Demyelinating Diseases including: 1. Multiple Sclerosis (MS) 2. Transverse Myelitis (TM) 3. Guillain-Barre Syndrome (GB)
Recent research supports using Ivermectin in demyelinating diseases such as Multiple Sclerosis. There are more papers supporting the role of Ivermectin in neurological conditions: Ivermectin promotes peripheral nerve regeneration (2018 Cairns et al) Ivermectin protects neurons & prolongs lives of ALS mice (Amyotrophic Lateral Sclerosis) (2007 Andries) Ivermectin has been proposed as a treatment for Parkinson’s Disease (2021, Dongwook Wi, Master’s Thesis) Ivermectin protects the brain of rats during a stroke, reduces infarct size, improves memory and learning of rats with cerebral ischemia-reperfusion (2023 Seyyedabadi)
https://x.com/MakisMD/status/1809540107377971235
COVID-19 BIOWEAPON² PART 1 Executive Summary https://bit.ly/3Fuiwdu
Written: March 2020
BIOWEAPON 1 – virus
BIOWEAPON 2 – shots
COVID-19 BIOWEAPON² PART 2 https://bit.ly/3eI11bK
COVID-19 BIOWEAPON² PREREQUISITE FOR U.S. INVASION – LONG TERM PLANNING
https://tinyurl.com/37fbrevd
COUNTERMEASURE “VACCINE” – NOT EFFECTIVE, NOT SAFE, NOT MADE AT “WARP SPEED”
https://tinyurl.com/5n7tajc5
CHILDREN SHOULD NOT GET THE “VACCINE”
“ABOVE ALL DO NO HARM”
Written November 21, 2021 https://bit.ly/3nB4CyK
PROTECT YOURSELF FROM THE SPIKE: COVID-19 INFECTION and/or VACCINE – OUR NEXT HEALTH CRISIS
Written August 23, 2021 https://bit.ly/3kkrejY